SPRI Bead to Sample Ratio for DNA Cleanup and Size Selection
What is the Bead to Sample Ratio?
The Bead to Sample Ratio, also known as the Bead Ratio, is an essential factor in DNA cleanup and size selection using SPRI bead-based reagents such as AMPure XP, SPRIselect, or RNAClean XP. It refers to the ratio between the volume of the bead-based reagent and the volume of the nucleic acid sample.

AMPure XP Bead Ratio for DNA Cleanup
AMPure XP reagent, powered by SPRI technology, is used for PCR purification and next-generation sequencing (NGS) cleanup. It ensures high recovery of amplicons, greater than 100 bp, and efficient removal of unincorporated dNTPs, primers, primer dimers, salts, and other contaminants.In DNA cleanup, the recommended bead ratio for AMPure XP is 1.8. This value denotes the volume of the AMPure XP bead required in relation to the volume of the DNA sample. To determine the volume of AMPure XP reagent per reaction, multiply the volume of the DNA sample by 1.8:
Changing the AMPure XP bead ratio value will affect the length range of DNA fragments in the output sample (Fig. 1). Some suppliers have developed DNA size selection protocols using the AMPure XP reagent. However, we recommend using our SPRIselect reagent for size selection, which is purposefully validated for this application.



| CHART COLOR | Bead/Sample Ratio |
| PINK | Input/Reference |
| RED | 1.8X |
| BLUE | 0.9X |
| GREEN | 0.7X |
| AQUA | 0.6X |
Figure 1. Agilent TapeStation system traces of sheared gDNA purified using AMPure XP reagent, Reagent “K”, and Reagent “O”. Cleanups were performed using 1.8x (red), 0.9x (blue), 0.7x (green), and 0.6x (aqua) bead to sample ratios as shown. Traces show relative differences in peak height and recovery efficiency.
Comparing the traces for different reagents, we can see that only AMPure XP reagent provides predictable performance while maintaining efficient recovery, proven by the relative difference between the reference and 1.8X ratio in the traces.
SPRIselect Bead Ratio for DNA Size Selection
SPRIselect reagent offers enhanced flexibility and control during the DNA size selection process. It’s widely used in fragment library preparation for NGS, as the size of DNA directly impacts the desired sequencing results.
SPRIselect reagent enables scientists to perform various types of size selection:
- Left Side Size Selection: This method removes DNA fragments that are smaller than the targeted size, allowing for the selection of larger fragments.
- Right Side Size Selection: Conversely, this method removes DNA fragments that are longer than the targeted size, enabling the selection of smaller fragments.
- Double Size Selection: This approach involves DNA fragments within a specific range, ensuring that they fall between a minimum and maximum size (no smaller than 150 bp and no larger than 800 bp).
The bead ratio, used in the DNA size selection protocol, depends on side of selection and selection points:


To learn more about bead ratios used in double size selection protocols, please refer to the SPRIselect User Guide.
The Impact of Switching to a Kit with a Different Ratio
It is important to note that not all bead-based cleanup reagents utilize the 1.8x bead ratio. As shown in Figure 3, out of 12 different cleanup kits from various manufacturers, only 5 suggest the use of the 1.8x bead ratio for cleanup including AMPure XP and SPRIselect reagents.
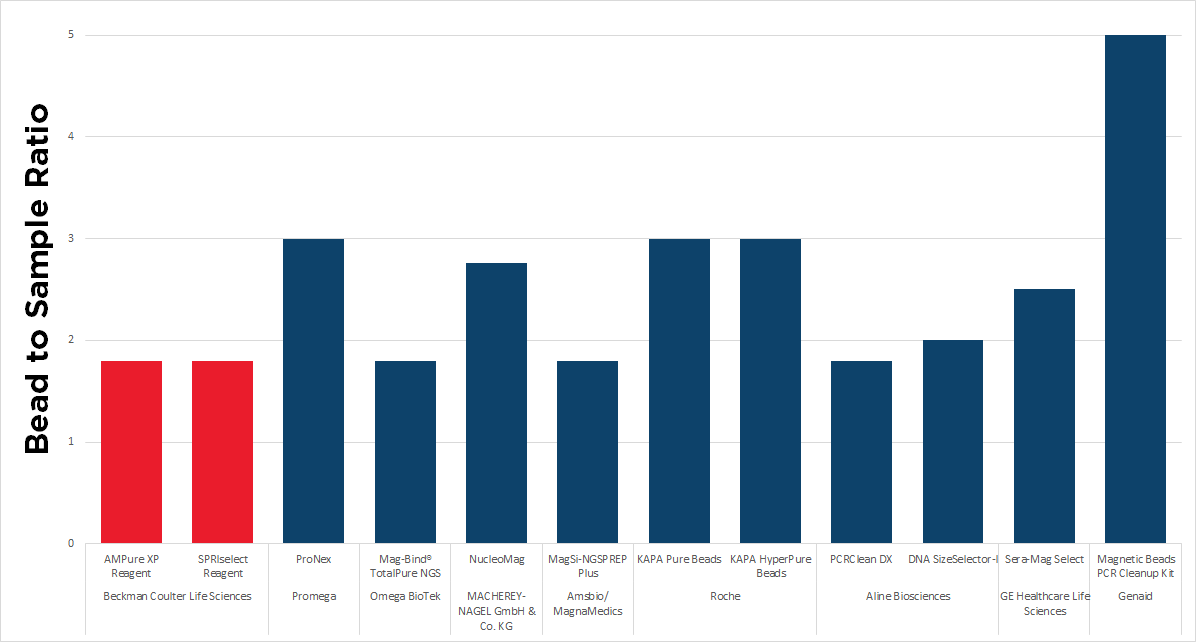
If you are contemplating switching to a different cleanup reagent, it is essential to consider potential consequences, which may include:
- Loss of Fragment of Interest: Different bead ratios can influence the efficiency of binding and recovery of specific DNA fragments. Switching to a kit with a different ratio may result in the loss of fragments that are of particular interest to your research.
- Recalculation of Bead Ratios: With the adoption of a new cleanup reagent, bead ratios will need to be recalculated based on the specific recommendations provided by the manufacturer.
- Rewriting Lab Protocols: Laboratories that previously used the standard 1.8x bead ratio in their protocols will need to update and modify their instructions to reflect the specific bead ratio associated with the new cleanup reagent.
Didn't find the information you need? Connect with one of our SPRI bead experts.
Talk to an Expert
Products and demonstrated applications are not intended or validated for use in diagnostic procedures.



