Minimizing Waste in Flow Cytometry Workflows
Many clinical cytometry laboratories across the globe are challenged with test numbers increasing while budget and head count remain constant. This ultimately drives a continuous need for optimized workflows and the elimination of waste.
In order to optimize and standardize workflows, labs need to identify the common sources of waste in their processes, and reduce them to free time for other productive work. Many labs believe they are efficient, having previously drilled down their operations and processes. However, efficiency and the elimination of waste requires continuous improvement and is not a one-off event.
Transitioning lean manufacturing practices to the clinical lab demonstrates that even with the most efficient lab, there is always an opportunity to remove further waste and improve the overall efficiency of current processes.
The 8 Wastes
The 8 wastes are a concept that originates from Toyota Lean Management, and today it is increasingly used outside the original manufacturing context. The commonly used acronym for the 8 wastes of lean manufacturing is DOWNTIME, which stands for:
- Defects: Efforts caused by rework, scrap and incorrect information.
- Overproduction: Production that is more than needed or made before it is needed.
- Waiting: Wasted time waiting for the next step in a process.
- Not utilizing talent: Underutilizing people’s talent, skills and knowledge.
- Transportation: Unnecessary movement of products and materials.
- Inventory excess: Excess products and materials being processed.
- Motion waste: Unnecessary movements of people (e.g., walking).
- Extra processing: More work or higher quality than is required by the customer.
It provides a systematic approach to reviewing workflows: Are you maintaining too much inventory, are you making defective products, are you moving it around too much? Taking this established approach and transferring it to the clinical laboratory setting, it can be used to investigate the inefficiencies of the routine clinical flow cytometry workflow.
Defects
In the routine clinical cytometry lab, many defects can be attributed to human error. The typical areas of defects consist of adding a wrong reagent, preparing a cocktail incorrectly, transcription errors or samples being switched.
Overproduction
One way to speed up sample preparation is by preparing pre-mixed antibody cocktails. However, if future sample requests are overestimated, a proportion of the pre-mixed reagent cocktails will be discarded before they can be used.
Waiting Times
Sometimes some of the processes in your lab are outsourced due to resource limitations, and that would involve a waiting time. Key areas where waiting is part of your current lab workflow:
- Batch processing bottlenecks
- Centrifugation and incubation times
- Send-out test TAT (turnaround time)
Non-utilized Talent
This is considered a very specific type of waste, and one which should never be ignored. In this situation, you have highly skilled staff performing low-skill tasks. In addition, there is an opportunity cost, as introducing new or improving existing panels doesn’t occur because highly skilled staff are occupied with pipetting or dealing with inventory management.
Transportation
Transportation is not only a process waste, but also a threat to the environment. Many labs struggle to manage their inventory, as they are often challenged by staggered expiry dates for the reagents they source. It should be considered that each reagent order can require multiple temperature-controlled deliveries. Moreover, the need to outsource testing also comes with the requirement for additional transportation of samples.
Inventory
Multiple deliveries of single-color reagents from different vendors make inventory management tedious and complex due to varying quality and staggered expiry dates. This can lead to scrapping of expired reagent and cocktails.
Motion
In the lab there are many times when staff must move away from the workbench to a refrigerator or centrifuge. If you are opening and closing centrifuges or refrigerators all day long, that is unnecessary motion.
You know there are too many pipetting steps and opening of your reagent containers. If you are pipetting, that's unnecessary motion. If you are having to transcribe, that's unnecessary motion. All these motions consume time.
Extra Processing
Extra processing or repeat testing is required when errors occur during any part of the lab workflow. Identifying and eliminating the common sources of error improves turnaround time and efficiency.
Assessing Your Waste
Observe
The key to identifying waste is observation, which involves a visit to the place of work to watch the workflow and what people are doing. Talking to people about the workflow or reading a procedure manual will not identify areas of waste.
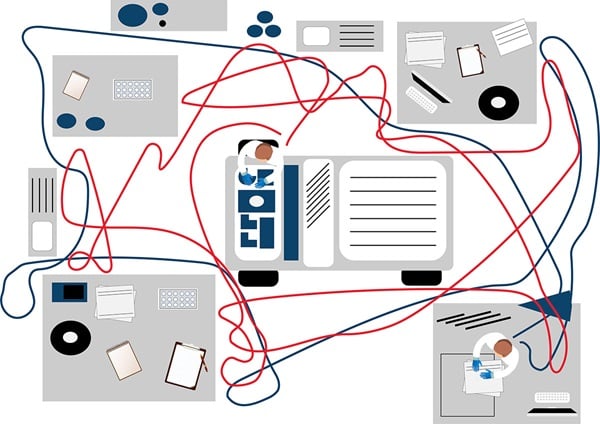
A popular tool to illustrate workflows is the spaghetti model or a spaghetti diagram, which shows a hypothetical manufacturing floorplan with two employees (Figure 1a). The aim is to capture their paths of movement, which is done by sitting in the lab and observing where staff go, even if it is a repeated movement.
Figure 1a. The spaghetti model shows a hypothetical laboratory floorplan and two employees.
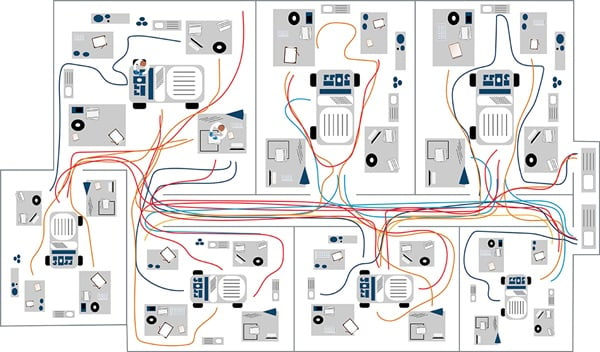
Figure 1b. Example clinical cytometry workflow spaghetti model.
In the example spaghetti diagram for a laboratory, the colors represent individual lab staff and their respective movements. The example shows individuals marked in blue spend the most time in one area. Comparing this to the brown line, you can see this individual makes repeated trips to another room—this presents an opportunity to reduce potentially wasteful movement (Figure 1b). Another approach to the spaghetti model is to follow a sample along its workflow.
Quantify
The next step that will help you to understand your workflow is to quantify it by identifying measurable steps:
- How many minutes does it take?
- How many minutes do we wait?
- How many steps does it take?
- How much travel is required (measure in footsteps or tiles on the floor)?
- How many pipetting steps?
- How much inventory is on hand?
This establishes a baseline and enables quantification of any workflow improvements implemented.
Prioritize
Once areas of waste are identified, they can then be prioritized by Pareto analysis (ranking them from high to minimal impact). This enables identification of areas with the biggest impact to be addressed first, also known as the 80/20 rule (i.e., 80% of consequences come from 20% of causes).
Figure 2. Hypothetical example of a Pareto chart for a clinical flow cytometry lab.
The point where the cumulative percentage exceeds the 80% mark indicates the areas of waste that are low priority and should not be the current focus. Based on the findings, you would then want to implement countermeasures and sustain/track them relative to whatever factor was identified earlier as something measurable to track against.
Streamlining Workflows in Clinical Cytometry
Now we will illustrate this with an example workflow in a clinical cytometry lab that builds on the spaghetti diagram in Figure 1a.
To develop a test, the lab purchases several separate single-color reagents and combines them to create a test that generates the desired results.
Typically, the steps involved in this testing workflow include:
1. Inventory Check
Check the inventory of single-color antibodies and discard those that are beyond their expiration date. Antibodies are stable for a limited period, and each will have a shelf-life date that must be documented. If reagents need to be discarded because they have exceeded their expiration date, then additional single-color antibodies must be reordered, and the lab must wait for them to be delivered. Once delivered, the antibodies and their location, expiration date and when they were opened etc., must be documented.
Figure 3. Workflow inventory check.
2. Creating a panel
When it's time to start testing, lab staff select the single-color antibodies they need, move them from the fridge to the bench and open all the lids. After that, many labs still pipette their single-color reagents manually.
In this workflow, assume the lab is creating a 10-color panel (which is 10 antibodies per tube, as it is 10-color flow cytometry). As an example, to create the panel, four tubes are required (the total number of tubes will depend on the actual panel). The process has many sources of waste; however, for illustration purposes we will focus on the waste around the last step of pipetting the single-color antibodies, as it is a time-consuming, manual and error-prone step. When done manually, it is repetitive motion, as lab staff must pipette each antibody individually, for a total of 40 pipetting steps, in addition to uncapping and recapping bottles.
Figure 4. Workflow inventory check and 10-color panel creation.
3. Countermeasure examples
Instead of lab staff pipetting all reagents one-by-one for every single sample, a potential countermeasure is to prepare cocktails of single-color antibodies (Figure 5). In this illustration, we prepare four different cocktails for 5 patient samples. This equates to one cocktail per four tubes and would reduce the number of pipetting steps from 200 (40 antibodies multiplied by 5 samples) to 60 (4 cocktails with 10 antibodies each plus dispensing of cocktails for 5 samples) —a significant improvement with less opportunity for error and reduction in repetitive motion.
However, adding this step to the workflow does create additional work preparing the multicolor cocktails of single-color antibodies using liquid reagents. This would require additional quality control (QC) and, depending on case load, may result in the discarding of expired cocktails.
In addition, preparing multicolor cocktails with single-color antibodies does not fix the early part of the workflow. The workflow concerning receipt of single-color antibodies is cumbersome and is still not addressed. An alternative approach is reducing the tubes, again from four tubes to three tubes. This can be done by switching from a ten-color flow cytometer to a 13-color cytometer or by stacking markers within the panel.
Figure 5. Workflow inventory check and multicolor cocktail workflow for creation of 10-color panel.
Alternative approaches to reducing waste include removing the reliance on single-color liquid reagents or installing an automated sample preparation system to expand laboratory testing capabilities, especially those focusing on complex lab-developed tests with mixed workflows. However, each lab should choose an approach that best fits their needs.
Summary
Efficient workflows are extremely important in the routine cytometry lab, and several countermeasures can be implemented to optimize them.
- Look for the low-hanging fruit associated with laboratory testing, and engineer out the appropriate manual and transcription steps. These are always associated with labor-intensive processes and are major sources of error.
- Investigate ways to save time by simplifying inventory management and decrease batch processing where possible.
- Maximize your multiparameter capacity, allowing you to do fewer tubes and decrease labor at the same time.
- Having fewer tubes leads to less antibody redundancy and reduced reagent usage.
- Use the concept of stacking markers and get even more markers in a tube, thereby maximizing multiparameter capacity.