Evaluation of the Analytical Performance of the AQUIOS CL Flow Cytometer in a Multi-Center Study
Beckman Coulter Diagnostics, Inc., 1Clinical Research, Miami, Florida, USA
2London Health Sciences Centre, London, Ontario, Canada
3The University of Texas Medical Branch, Galveston, Texas, USA
4University of Miami Miller School of Medicine, Miami, Florida, USA
Beckman Coulter, Inc., 5Clinical Quality Assurance, Research and Development, Miami, Florida, USA
Beckman Coulter Life Science, Inc., 6Research and Development, Miami, Florida, USA
Introduction
A multi-center study was conducted to establish the performance of the AQUIOS CL Flow Cytometer with AQUIOS Tetra Panel reagents for lymphocyte subset analysis in patients suspected of immunodeficiency, post-transplant patients and apparently healthy individuals. Performance equivalency was evaluated compared to the BD FACSCalibur™ Flow Cytometer with Multitest™ IMK kit and TruCount tubes.
Objectives and Methods
Precision performance of the AQUIOS Tetra system was evaluated based on CLSI EP5-A2 using control materials as samples.
To assess substantial equivalency, the AQUIOS CL Flow Cytometer with AQUIOS Tetra reagents was compared against the BD FACSCalibur™ Flow Cytometer with BD Multitest™ reagents using a single platform approach for absolute counts. A multi-center study was conducted to target the intended use population following CLSI EP09-A3. The AQUIOS Tetra gating method was compared to the FACSCalibur™ system with Multiset™ software in ≥400 samples covering the CD4+ medical decision levels. Fully automated sample preparation was performed by the AQUIOS CL Flow Cytometer and manually for analysis on the FACSCalibur™ Flow Cytometer.
Hematologically normal adult donors between 18—65 years old with a targeted ratio of 50:50 males to females from three (3) geographical locations were combined to establish the reference interval for AQUIOS Tetra-1 Panel and Tetra-2+ Panel. The adult reference interval was determined following CLSI EP28-A2.
Results
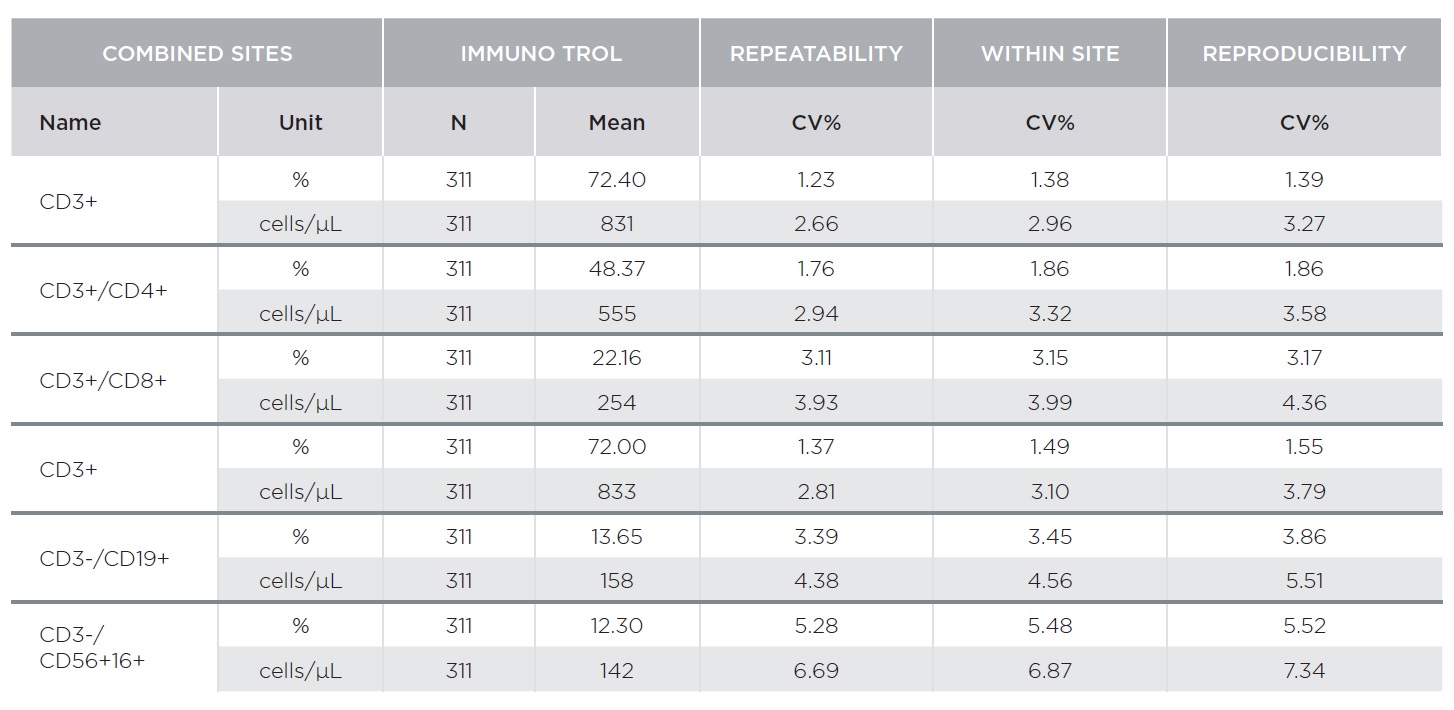
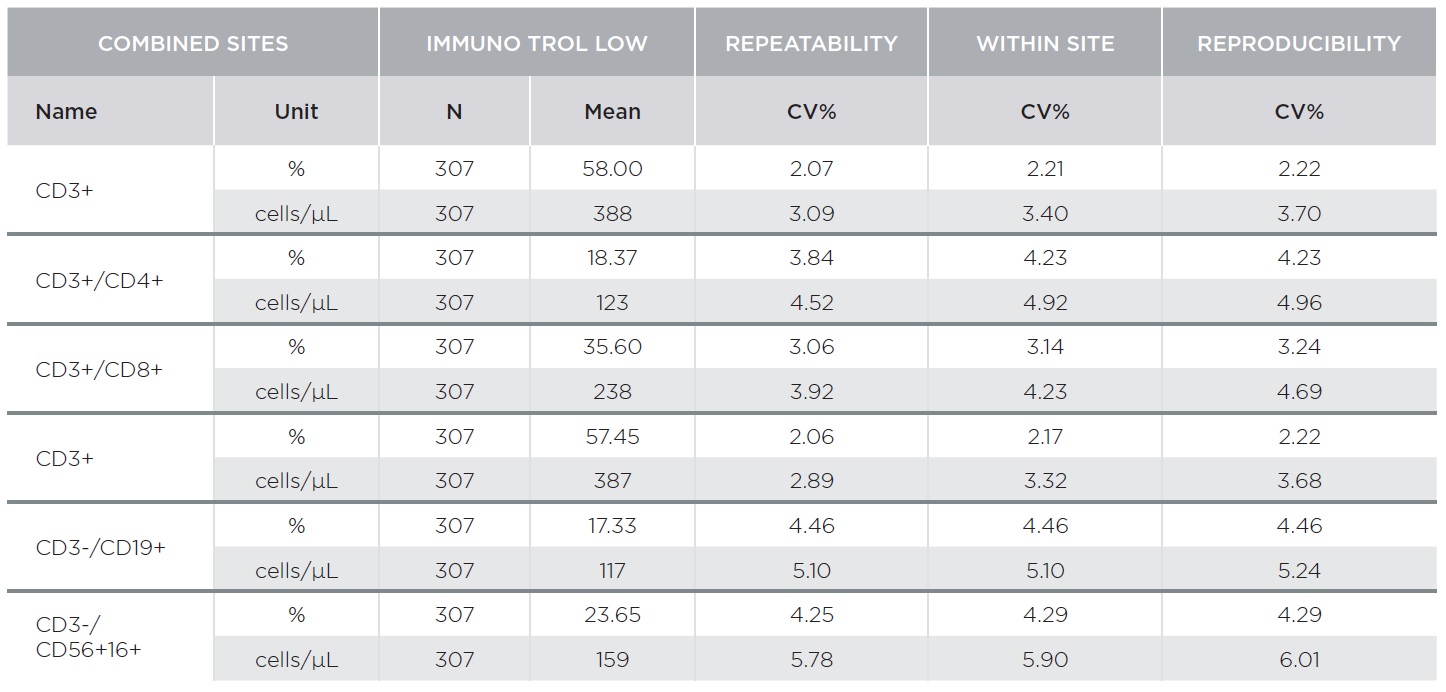
The AQUIOS CL Flow Cytometer showed excellent repeatability and reproducibility. Tables 1 and 2 summarize representative results for AQUIOS Tetra-1 and AQUIOS Tetra-2+ reagents for all sites combined. Two levels of control material were evaluated at three (3) sites. Tables 1 and 2 provide results for AQUIOS IMMUNO-TROL Cells and AQUIOS IMMUNO-TROL Low Cells, respectively. The repeatability and reproducibility for very low CD4 levels (123 cells/μL) was <5%.
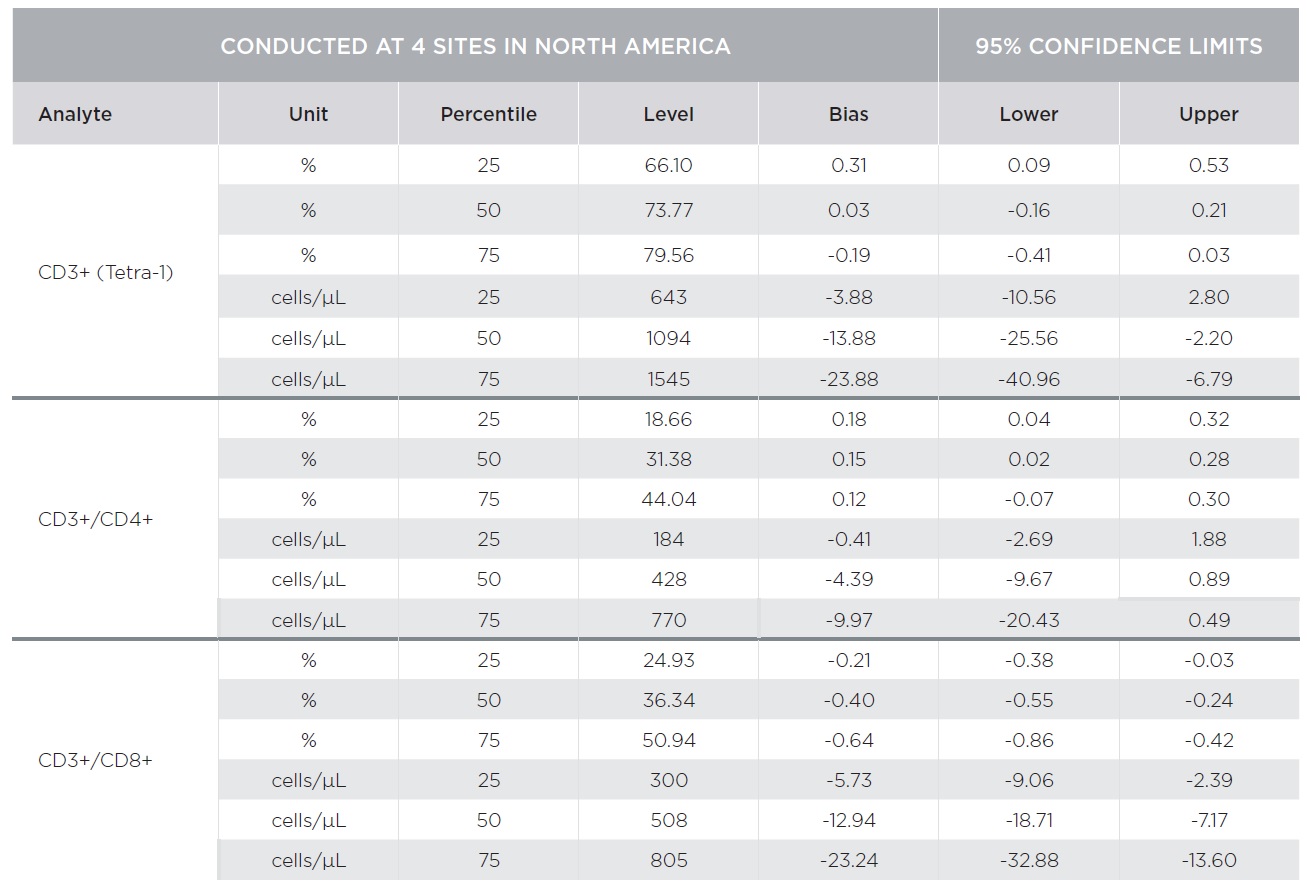
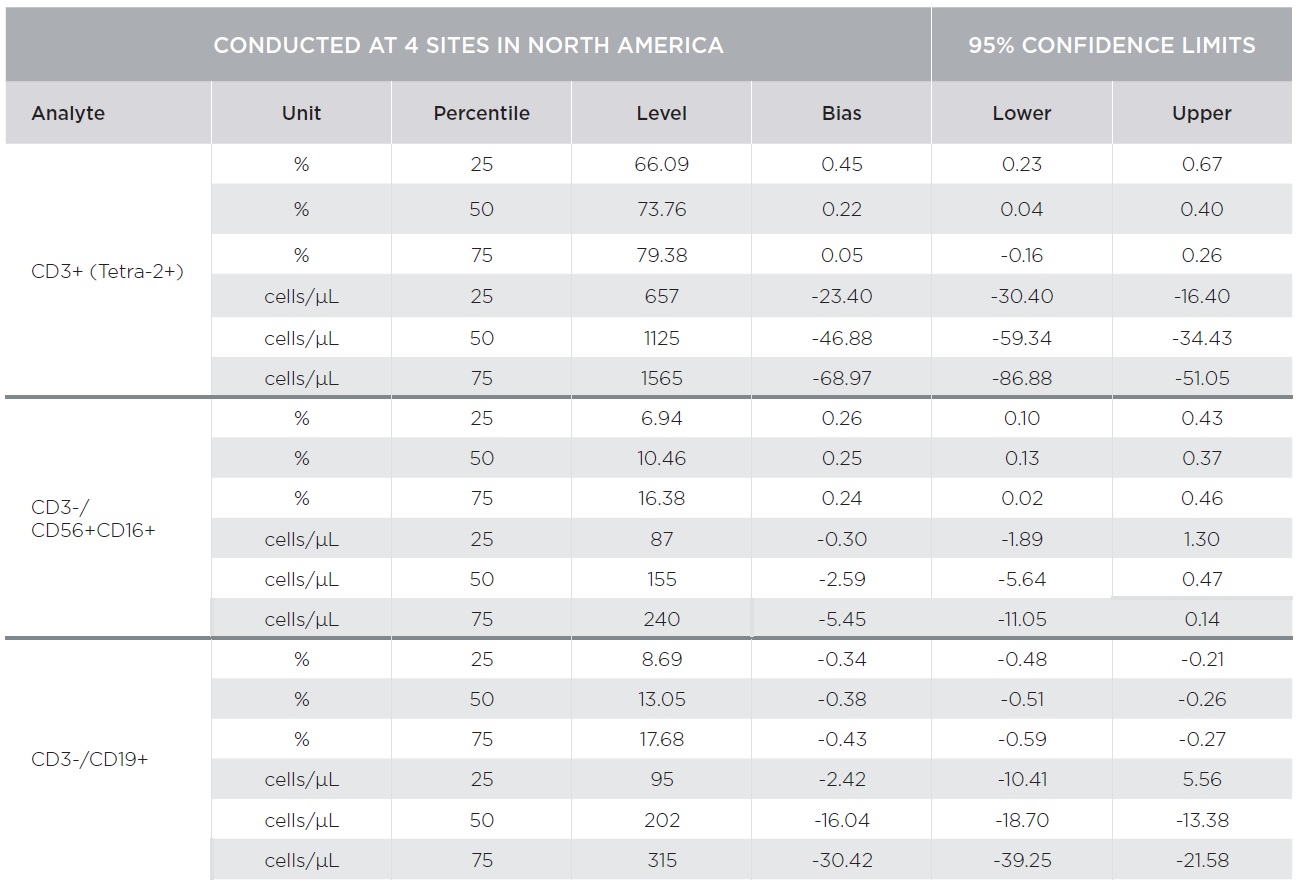
Substantial equivalency of the AQUIOS CL system with AQUIOS Tetra reagents was demonstrated against the BD FACSCalibur system with BD Multitest™ reagents [CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC and CD3-FITC/CD16+CD56-PE/CD45-PerCP/CD19-APC] using a single platform approach for absolute counts [TruCount]. A total of 443 samples combined from four (4) clinical sites covering the CD4 analytical measuring range with emphasis on the medical decision ranges were tested on both platforms.
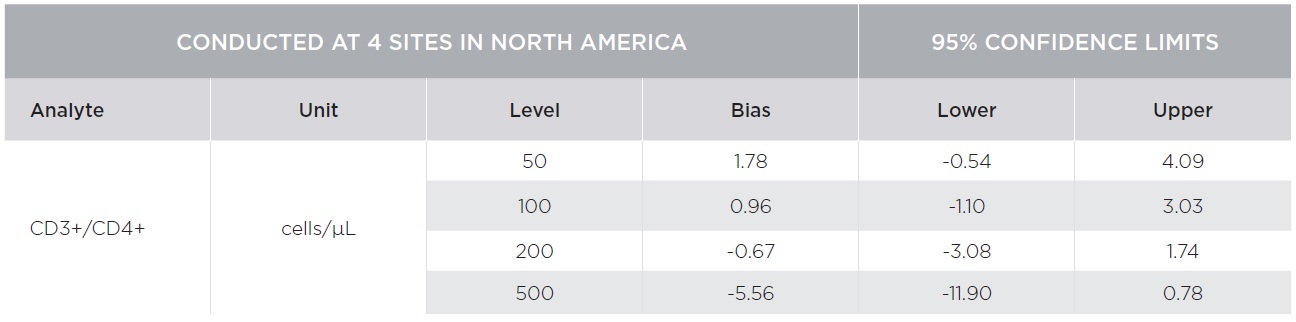
Percent marker recovery comparisons had clinically insignificant to no bias. A negative bias for the comparison of the AQUIOS system to the BD FACSCalibur™ system was observed in the absolute count marker recovery for all four (4) sites combined. This negative trend observed for all markers was not clinically significant. Smaller differences were observed for CD3+/CD4+ counts compared to other markers as summarized in Tables 3 and 4. The bias was clinically insignificant at the medical decision points for CD3+/CD4+ as noted in Table 5. The additional parameters that AQUIOS provides (CD45+ and CD45+ Low SS) are not provided.
Figures 1 and 2 illustrate regression and Bland-Altman plots, respectively for CD3+/CD4+ count confirming comparability of AQUIOS Tetra application to the BD FACSCalibur™ Flow Cytometer.
A total of 161 hematological normal adult donors were combined to establish reference Interval. Normal reference intervals for lymphocyte subsets (data not shown) were consistent with published values.
AQUIOS Tetra Precision Performance: AQUIOS Tetra-1 Panel [CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5] and AQUIOS Tetra-2+ Panel [CD45-FITC/(CD56+CD16+)-RD1/CD19-ECD/CD3-PC5]
AQUIOS Tetra (Test) vs. BD FACSCalibur (Reference): AQUIOS Tetra-1 Panel [CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5] and AQUIOS Tetra-2+ Panel [CD45-FITC/(CD56+CD16+)-RD1/CD19-ECD/CD3-PC5]
Conclusions
The AQUIOS CL flow cytometer demonstrated excellent performance in lymphocyte subset analysis with AQUIOS Tetra Panel Reagents.
AQUIOS CL is a Class I Laser Product.