Basics of Clinical Flow Cytometry Webinar
Leveraging existing HIV laboratory capabilities to transform cancer diagnosis in Sub Saharan Africa
African Organization for Research and Training in cancer virtual educational workshops symposium
For many years, Beckman Coulter Life Sciences has recognized the need to provide and support humanitarian aid for low middle-income countries. Over the last five years, in collaboration with physicians and scientists at Burkitt’s Lymphoma Fund for Africa (BLFA), as well as local physicians of Surgery personnel in Sub Saharan Africa, we have launched the CARES Global Health Initiative. As part of this effort, we build alliances and partnerships that establish greater access for clinical testing. Led by Tony Boova the CARES Initiative supports equity for health access through multilateral partnerships that increase laboratory capacity and helps realize life's potential within the poorest countries in the world.
Burkett’s Lymphoma Fund for Africa (BLFA) is a US based pediatric cancer nonprofit and we are working with partners in Africa and collaborators such as Beckman Coulter Life Sciences to improve the survival rates for children with cancer.
Steve is a BLFA board member and is leading the charge for our pathology improvement initiatives. He is also managing director of PhenoPath Laboratories, a Quest Diagnostics company in Seattle, WA and he will be speaking to us today about the basics of clinical flow cytometry and how we apply it to the diagnosis of cancer.
Flow cytometry and how we apply it to the diagnosis of cancer
I will walk you through what we are trying to do from a technical point of view and introduce everybody to flow cytometry and how we apply it to the diagnosis of cancer in pediatric patients that are a particular focus of the BLFA, but also in adult patients as well.
I will start by describing what goes on in a flow cytometer. We all know about flow cytometry and certainly Beckman Coulter's been involved in this for years in Africa in terms of HIV monitoring, CD4 positive T cell counting, but we can apply that same technology to diagnosing cancer, particularly blood related cancers.
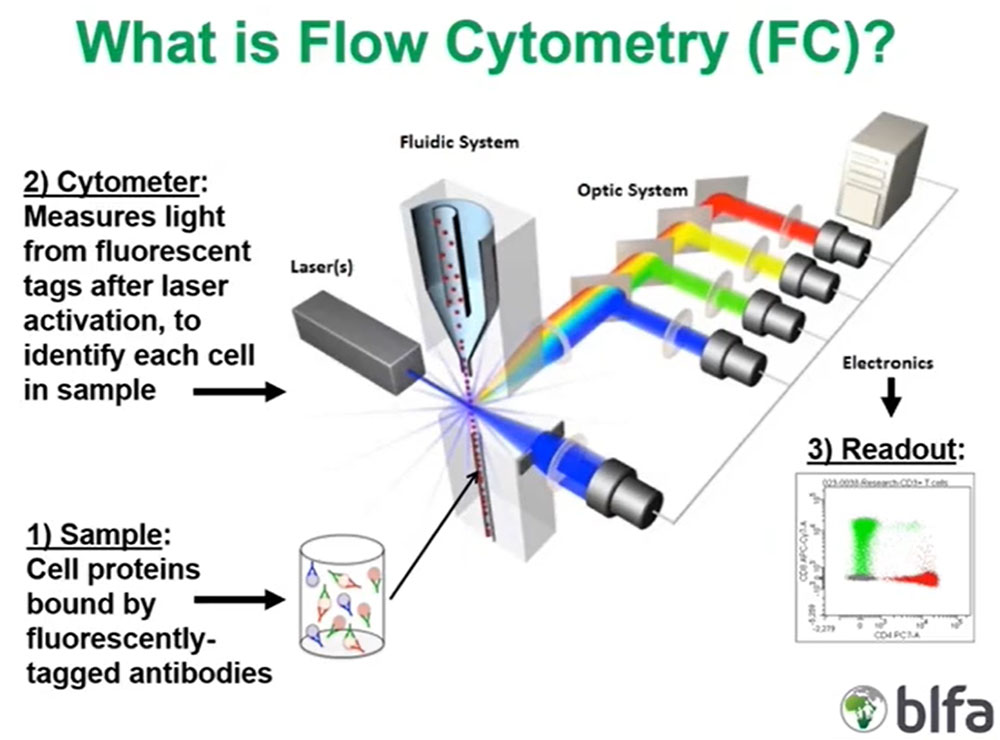
Let us start with the flow cytometry sample. Flow cytometry is a way to evaluate living cells in suspension, and those can be cells derived from blood or bone marrow or body fluids or tissues. Those cells are labeled by fluorescently tagged specific antibodies to antigens either on the cell surface or inside the cell, and that labeled mixture of cells and antibodies is then introduced into the flow cytometer via what we call the fluidic system.
What is critically important is that those cells are assembled in a single file manner in the cytometer due to the way that the fluid in the cytometer is introduced and that single file organization of cells is then illuminated by one or more lasers, which are light sources of a very defined single wavelength.
The lasers excite the fluorescent molecules on the surface of those bound antibodies on the surface or inside the cell, and they emit certain wavelengths of light that are then partitioned by the optical system to different detectors that measure those specific wavelengths.
What is critically important is those detectors will convert that optical information, that light energy, into electronic impulses that are then assembled by the flow, analysis software on the associated computer to the cytometer and converted to the two-dimensional scatter plots, or dot plots, the typical readout that all of us are used to seeing where we can then interpret what the antigens are that are being measured on those cells. And these are this is a shown here is just an analysis of T cells including some red CD4 positive T cells and some green colored CD8 positive cells and each dot on these readouts corresponds to an individual cell.
Measured Parameters in Flow Cytometry
There are two kinds of major measures of cell properties, one is just generic light scatter properties that all particles in solution have. The first of which is forward scatter or full forward angle light scatter that is proportional to particle or cell size, so larger cells have higher forward scatter.
Importantly, as cells start dying or degenerating their membrane integrity starts decreasing. They lose forward scatter so it is a useful way to exclude dead or dying cells from the analysis because we really do not want to look at those cells because they can introduce artifacts.
The second generic light scattered parameter we use routinely in the lab is side scatter or right-angle light scatter that is proportional to cytoplasmic complexity or granularity.
The second major fluorescent features of cells or are really cell associated fluorescence that are due to molecules that fluoresce. The first of those measures is intrinsic fluorescence or what we call autofluorescence that is due to the presence of large biological molecules in cells that when exposed to a light source will have their own fluorescence. That is something we just need to account for when we look at cells by flow cytometry.
The second and most relevant type of fluorescence is extrinsic fluorescence due to introduced substances that could fluoresce when exposed to the laser, and those could include DNA (deoxyribonucleic acid) binding dyes that allow us to determine if the cells have a normal or an abnormal amount of DNA, and most importantly for what will be talking about here or bound fluorescently labeled antibodies, that is all extrinsic fluorescence.
Flow Cytometry Advantages
There are several advantages to flow cytometry. The most important is that we can look at each individual cell in a complex mixture and get a lot of multiparametric information so we can look at a variety of different targets in the cell or we can look at the cell's DNA.
More importantly for the life-threatening diseases that all of us in the world of hematopathology deal with daily, flow is very rapid. From the time a sample arrives in the laboratory to the time we can render an interpretation of what we are seeing can be a matter of two or three hours. For acute leukemias and other life-threatening illnesses this is critically important. As we know from the world of HIV monitoring flow is both quantitative, reproducible and it is a flexible methodology. For a type of assay or a type of cytometer we can look at multiple different targets, reagents etc.
A real advantage in Sub Saharan Africa, and one of the reasons that my collaborators and I decided to use flowcytometry beyond HIV monitoring to help diagnose cancers, is that there was already infrastructure in place for using flow cytometry throughout Sub Saharan Africa. This allowed us to sidestep some of the obstacles that glass slide based traditional cancer diagnosis have.
Flow Cytometry Disadvantages
There are some disadvantages to flow cytometry that are worth keeping in mind. As we are looking at individual cells in solution and not looking at the whole tissue, so we lack that histologic context that we would get by looking at tissue biopsies under the microscope or blood or bone marrow smears. There are certain hematopoietic tumor types that are not easily identified, such as the malignant cells of classic Hodgkin lymphoma or may be underestimated, such as multiple myeloma plasma cells.
Obviously, we require viable material to make those suspensions from, that makes it a time sensitive technology with a need to process the sample the same day it arrives or potentially look no later than the following day. In my experience as a hematopathologist and among my colleagues in the world of hematopathology there is usually more expertise in glass slide-based diagnosis than there is in flow cytometry.
Clinical Flow Cytometry Assays
There are a variety of clinical flow cytometric assays:
- Lymphocyte subset analysis
- Immunodeficiency evaluation
- Stem cell enumeration (CD34)
- Reticulocyte enumeration
- Fetal erythrocyte identification
- H:LA cross matching
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Leukemia, lymphoma, and myeloma analysis
Our focus will be on leukemia, lymphoma, and myeloma analysis among this list of different asset types.
Unique aspects of leukemia, lymphoma, and myeloma flow cytometry analysis
Leukemia, lymphoma, and myeloma analysis has some interesting features in contrast to the enumerative assets like T cell subset testing. What we are seeking is abnormal populations with unknown immunophenotype's. As we look at the older population, the non-pediatric setting, there may be multiple abnormal populations that are related to having an older patient population with more diseases.
Importantly, not every abnormal population is clinically significant. We are aware that a variety of cancers have premalignant conditions such as monoclonal gammopathy of undetermined significance (MGUS) or monoclonal B cell lymphocytosis (MBL). These entities we need to be aware of but do not want to over diagnose as full-blown malignancy.
Optimal antigens to evaluate leukemia, lymphoma, and myeloma analysis
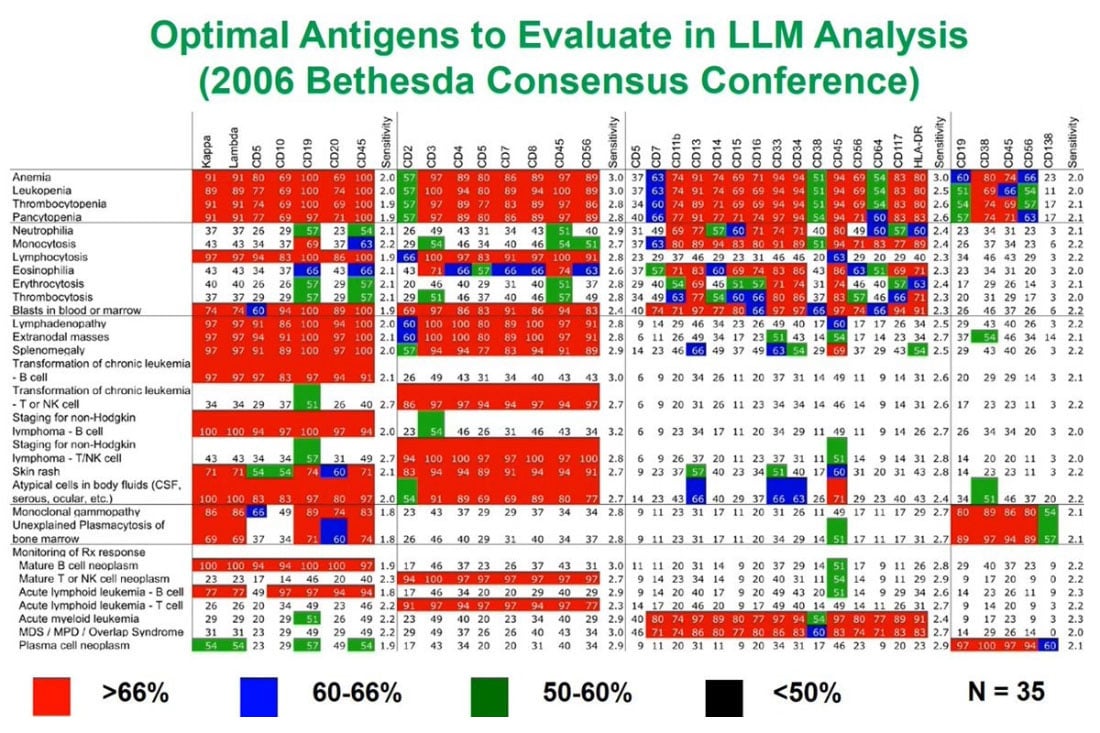
In thinking about the kinds of antigens we want to look at by flow cytometry, over the last 20 years a real consensus has arisen in the field which makes it easier for those who design assays to decide what to put in those assays.
I participated in the 2006 Consensus Conference in Bethesda, Maryland that produced a list for these indications along the left and what the antigens were most appropriate to look at. There are a group of B cell, T cell and myeloid associated antigens and plasma cell antigens. The red boxes indicate the ones where there was greater than two thirds consensus that they need to be part of any assay and the assays will discuss later incorporate a lot of these.
Basic of hematopoietic antigen expression: must understand normal to recognize abnormal
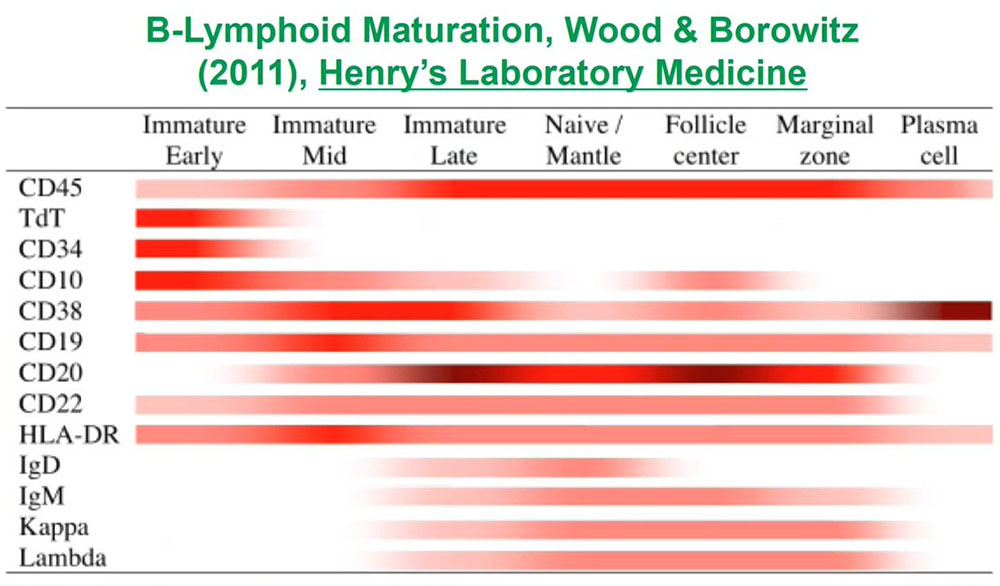
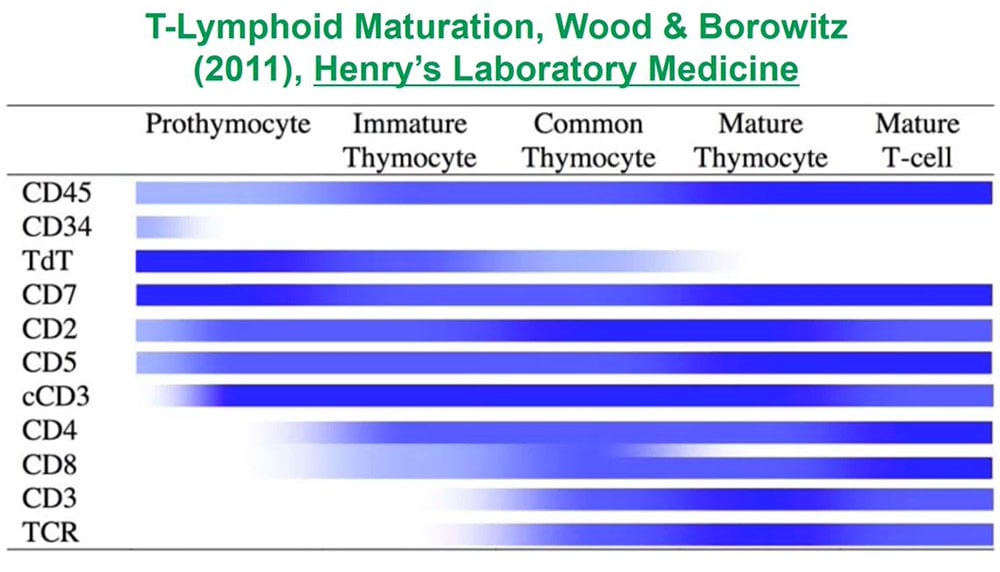
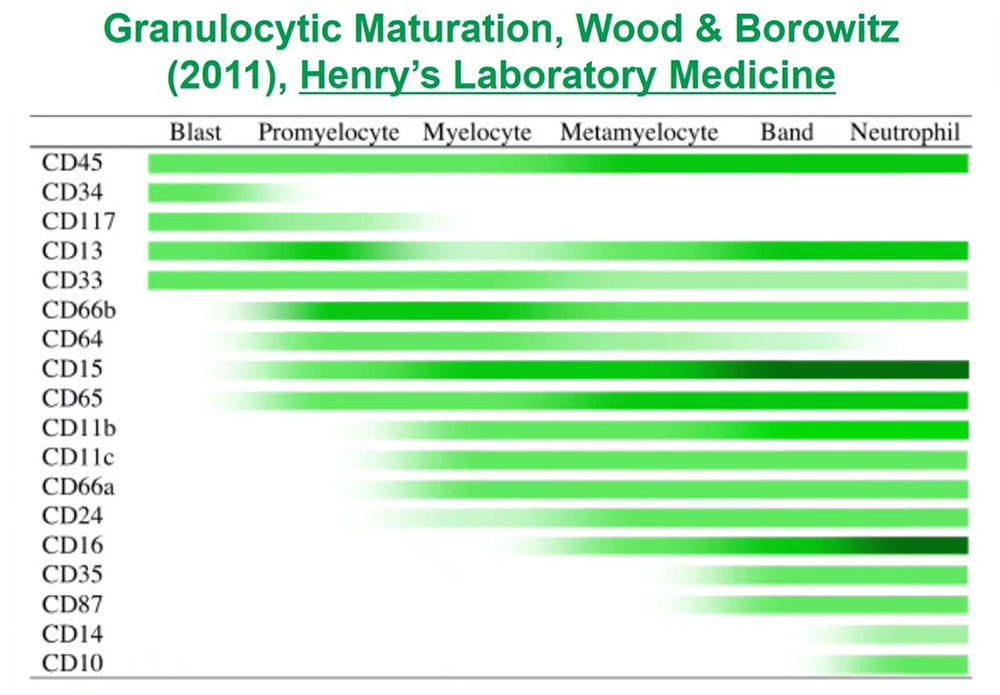
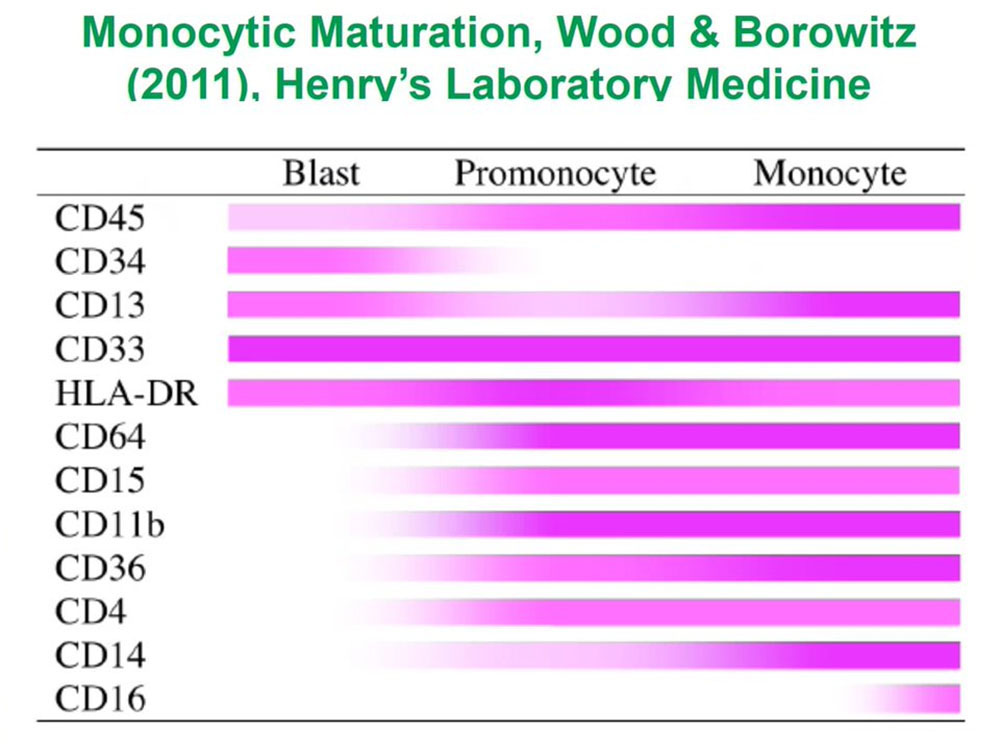
When we are looking for abnormal unknown populations, what is critical for those of us who analyze flow data is to understand normal antigen expression so that we can recognize abnormal populations as being different. To do that for each of the lineages for each of the types of cells in blood or bone marrow or lymphoid tissues we need to understand how those cells develop and how their patterns of antigen expression changed from the earliest immature precursors to the terminally differentiated forms.
For B cells which develop in the bone marrow, there is a whole list of antigens whose expression at different points during development we need to understand.
For T cells which develop in the thymus, similarly, there is a list of antigens we need to understand in terms of the normal timing of expression.
For granulocytes, which as a myeloid population developed in the bone again, we need to understand this large list of expression from the earliest progenitors or blast to the terminally differentiated forms.
For monocytes and other myeloid cell populations we need to understand the same sort of progression. And if we do that, if we understand all those patterns of antigen expression over time, we can look at complex mixtures.
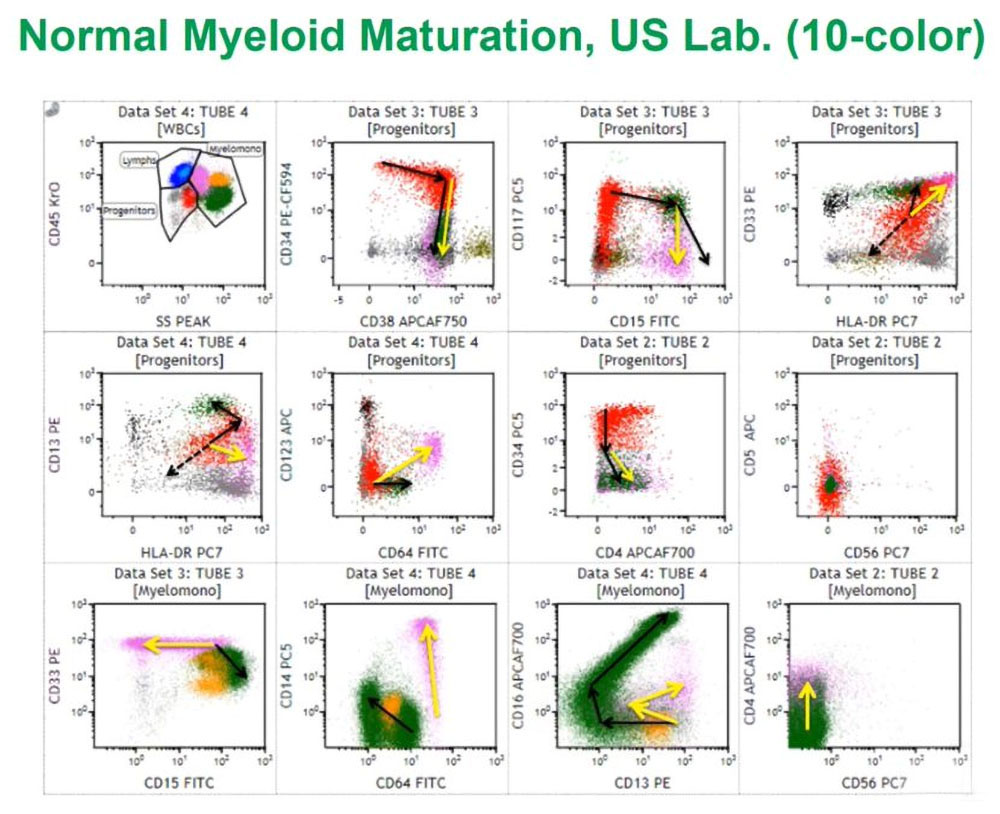
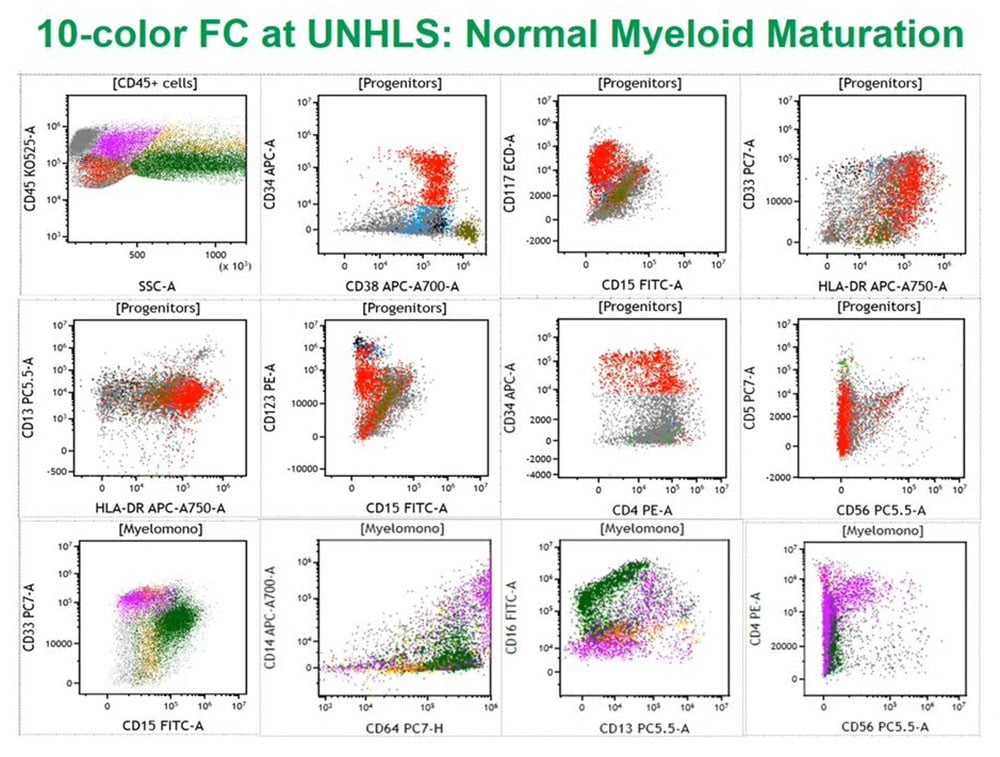
This is a normal bone marrow sample from my laboratory at PhenoPath that we looked at with ten color flow cytometry, using a similar assay to the one I will describe a little later. Using a variety of techniques, we first looked at CD45 or leukocyte common antigen against side scatter that measures cytoplasmic granularity we can partition the marrow into some distinct groups of cell populations and then look specifically at progenitors with the red colored cells always being CD34 positive myeloid progenitors and we can follow those arrows as they differentiate to either granulocytes or monocytes.
If we look at the granulocytes and monocytes, we always color the monocytes lavender pink and the granulocytes dark green as this is really a complex pattern recognition task so if we color things the same, we then see how those cells mature as monocytes or granulocytes. These patterns I have been looking at them for the last 25 years and they are very reproducible in normal marrows and make it easy to identify abnormal populations in the background of normal.
Leukemia, lymphoma, and myeloma cytometric assays leveraging the HIV experience
With my collaborators in Sub Saharan Africa, we have been leveraging the existing HIV flow cytometry experience to redeploy laboratories or create new laboratories that may be able to focus on leukemia, lymphoma, and myeloma testing. The two sites / geographic locations where we have done this are the Uganda National Health Laboratory Service, also called the Central Public Health Laboratory, in Kampala, Uganda and the Ampath Laboratory at the Maury teaching and Referral Hospital in Eldoret, Kenya.
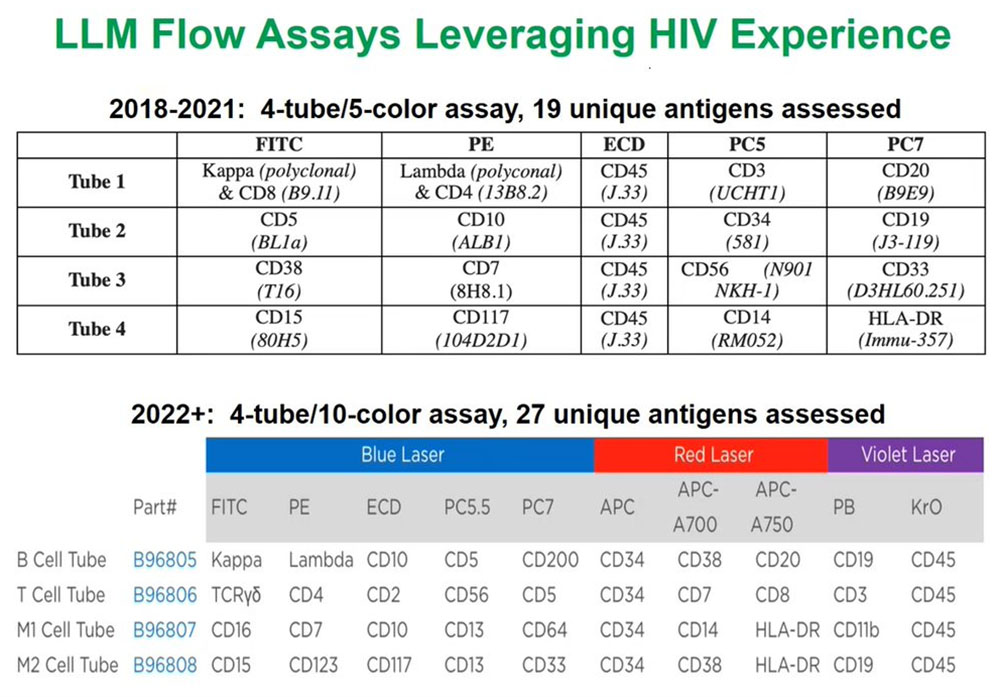
For the past three years we have deployed a four tube, five-color assay that allows us to measure 19 unique antigens and the list of antigens and the fluorochromes or the fluorescent tags on the antibodies of those antigens are shown here. This has been an especially useful assay that allows us to identify and help diagnose a wide variety of malignancies of all the different lymphoma, leukemia, and myeloma types.
But what we are excited to be deploying at both laboratories by the beginning of 2022, if not earlier, is another four-tube assay, but instead of looking at five antigens or five fluorescent molecules per tube we are now looking at ten. This exponentially increases the amount of information and can focus each of the tubes on specific cell lineages such as B cells, T cells, and then myeloid 1 and myeloid 2 cells and that enables us to use one or two of the tubes if we know that the population of interest is very defined.
UNHLS: Normal myeloid maturation 10 color cytometric data
This 10-color flow cytometry example is from the Uganda National Health Lab Service (UNHLS) that shows similar patterns to that seen in my lab with a slightly different assay. This confirmed to us in the validation process that the assay works well on these complex cell mixtures and we can also use it during the validation set.
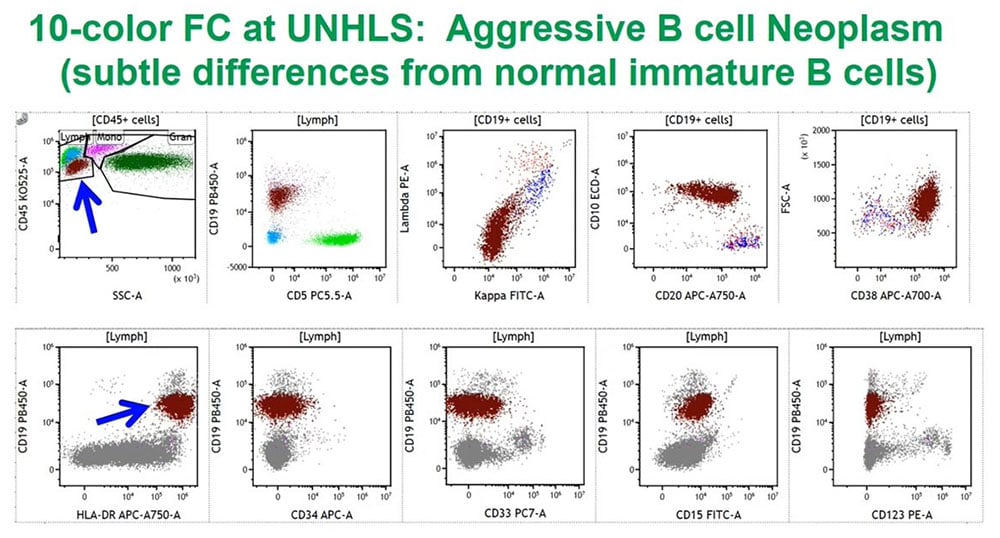
UNHLS: Aggressive B cell neoplasm 10 color cytometric data
We have looked at a lot of malignant samples and this example is an aggressive B cell neoplasm whose definitive diagnosis will require correlation with the glass slide-based tissue morphology. The maroon/rust colored cell population is the one of interest, and is from a young adult/adolescent male patient who is quite ill.
Expanding 10 color Flow Cytometry Services
Our plan for 2022 is to enhance the 10 color services. There are some additional assays to develop and deploy to expand the range of diagnosis we can make, such as the looking at cytoplasmic light chain expression in plasma cell neoplasms or helping identify ambiguous lineage acute taenias.
We are planning to introduce measurable residual disease (MRD) testing or previously called minimal residual disease (MRD)testing to look for small amounts of malignant cells post therapy, and that case will be evaluating 1,000,000 cells or more per tube to give us the sensitivity we want for the level of residual disease.
The UNHLS will help develop the ability to look at tissue biopsies and body fluid biopsies, which the latter is also not being routinely looked at the at the ARL lab. Which will expand the range of sample types where we can identify malignant populations and flow cytometry is helpful for looking at small numbers of malignant cells and cerebral spinal fluid (CSF).
We would like to develop a way for me to consult on cases for both labs by hooking up a camera to a microscope so we can generate a routine right stain smear, cytospin samples and take pictures so I can correlate the morphology with the flow findings. Several of my friends and acquaintances in the flow community in the United States and elsewhere have offered to help me in terms of serving as external consultants.
This has been a big effort over many years, and I would like to acknowledge my many collaborators: the UNHLS or Central Public Health Lab in Kampala. A really a large group of people and Dr Caroline Achola; the Ampath Group in Kenya; the clinician colleagues at the Uganda Cancer Institute; colleagues at PhenoPath including Vicki Ratliff who helped me develop the five-color assay.
My collaborators, my colleagues at the at the BLFA, and then finally Tony Boova and Eda Holl and our other collaborators at Beckman Coulter Life Sciences.